Critical Care Pulmonary
Pulmonary Embolism
A pulmonary embolism is a sudden blockage in the pulmonary arteries, typically due to a blood clot in the leg veins that breaks off and travels to the lungs. If this clot travels elsewhere, it’s called an embolus. Ignoring this condition can result in lung damage, reduced blood oxygen levels, or damage to other organs.
There are no major signs, but the basic symptoms that indicate this disorder include,
- Coughing up blood (hemoptysis)
- Shortness of breath on exertion or intermittently
- Chest pain that worsens with deep breathing and coughing
- Passing out (syncope)
Individuals having below-mentioned problems are likely to have PE.
- Advanced age
- Cancer
- Prolonged bed rest or immobility
- Blunt trauma
- Smoking
- Stroke or certain genetic conditions
- Estrogen-based medication or pregnancy
- Obesity
- After surgery
Diagnosis is typically based on a combination of:
- Symptoms
- D-dimer test (a blood marker for clotting)
- CT pulmonary angiography (CTPA) or lung ventilation/perfusion scan
Treatment typically involves anticoagulant medications, such as:
- Heparin
- Warfarin
- Direct oral anticoagulants (DOACs)
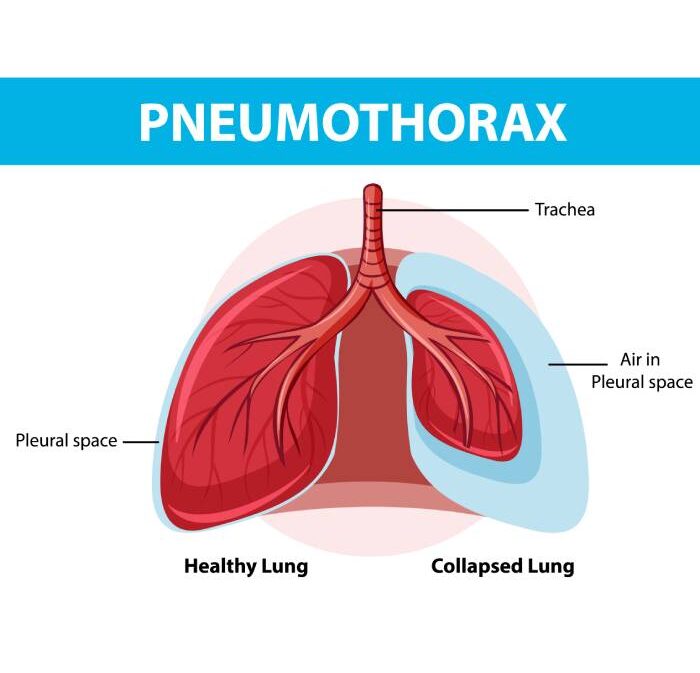
Pneumothorax
Pneumothorax, also known as a collapsed lung can be a minor issue or life-threathing condition depending on its severity. This occurs when air enters the space between the chest wall (pleural space) and the lung. As the air buildup, it puts more pressure on the lung and causes it to partially or fully collapse.
Spontaneous Pneumothorax
Occurs without any trauma or medical intervention, often in people with no known lung disease (primary) or those with underlying conditions like COPD or cystic fibrosis (secondary).
Traumatic Pneumothorax
This is caused by injury to the chest, such as from a car accident, a fall, or a puncture wound from surgery or medical procedures.
Tension Pneumothorax
A severe form of pneumothorax where pressure builds up in the chest, compressing the heart and other vital organs. It’s a medical emergency.
- Sudden sharp or stabbing chest pain
- Shortness of breath or difficulty breathing
- Rapid breathing and heart rate
- Fatigue
- Cyanosis (bluish color of skin due to lack of oxygen)
- A feeling of tightness in the chest
- Chest injury (blunt or penetrating)
- Lung disease (COPD, asthma, tuberculosis)
- Ruptured air blisters (in young, tall, thin men or smokers)
- Mechanical ventilation
- Certain medical procedures (e.g., lung biopsy, central line placement)
- Physical Examination: A doctor may listen for decreased or absent breath sounds on the affected side.
- Chest X-ray: The most common method for confirming pneumothorax.
- CT Scan: May be used in more complex cases to provide detailed imaging.
- Ultrasound: Increasingly used in emergency settings.
- Observation: For small pneumothorax, doctors may simply monitor the patient as the lung heals on its own.
- Needle Aspiration or Chest Tube: For larger pneumothorax, air may be removed with a needle or chest tube to allow the lung to re-expand.
- Surgery: In recurring cases or severe pneumothorax, surgery may be necessary to close air leaks or remove damaged lung tissue.
- Quitting smoking can reduce the risk of pneumothorax.
- Avoiding activities that might cause sudden pressure changes in the chest, such as scuba diving or flying without proper precautions.
- Patients recovering from pneumothorax should follow up with a doctor and avoid strenuous activities until the lung fully heals.
Acute Respiratory Distress Syndrome (ARDS)
ARDS is a severe, life-threatening condition involving fluid buildup in the tiny air sacs (alveoli) of the lungs. This fluid accumulation leads to widespread inflammation, impairing the lungs’ ability to provide oxygen to the body. ARDS typically affects critically ill or severely injured individuals and can progress to respiratory failure. It often arises from underlying causes, directly or indirectly damaging lung tissue.
- Pneumonia: Severe bacterial or viral infections, including COVID-19, can trigger ARDS.
- Sepsis: A widespread bloodstream infection that inflames the lungs.
- Trauma: Physical trauma to the chest or head.
- Aspiration: Inhalation of vomit, chemicals, or food.
- Pancreatitis: Inflammation of the pancreas causing systemic inflammation.
- Smoke or Chemical Inhalation: Exposure to toxic gases or chemicals.
- Near-drowning: Inhaling water damages lung tissue.
After an initial injury or illness, ARDS symptoms can develop rapidly within hours to a few days, including:
- Severe shortness of breath or dyspnea
- Rapid, shallow breathing
- Low blood oxygen levels (hypoxemia) despite oxygen therapy
- Cough and fever (if pneumonia is present)
- Fatigue or confusion due to oxygen deprivation
- Cyanosis (bluish tint on lips, nails, or skin)
Diagnosing ARDS requires several tests to assess lung function and rule out other conditions:
- Chest X-ray: Reveals fluid in the lungs.
- CT Scan: Offers detailed lung imaging.
- Blood Tests: Checks oxygen levels and identifies infections.
- Echocardiogram: Rules out heart failure as the fluid cause.
Treatment focuses on supporting breathing and addressing underlying conditions. ARDS patients usually require intensive care, and some may need long-term support:
- Oxygen Therapy: For extra oxygen; mechanical ventilation in severe cases.
- Mechanical Ventilation: When lungs cannot provide adequate oxygen independently.
- Prone Positioning: Improves oxygenation by redistributing fluid.
- Medications: Antibiotics for infections, anti-inflammatory drugs, sedation, and pain relief.
- Fluid Management: Balances fluid levels to reduce lung fluid.
- ECMO (Extracorporeal Membrane Oxygenation): In extreme cases, takes over lung function by oxygenating blood outside the body.
ARDS is serious, and recovery can be slow even with aggressive treatment. Some patients may experience long-term lung damage, while others may recover fully. Mortality risk varies with condition severity and underlying health issues.
- Lung scarring (pulmonary fibrosis)
- Weakness and fatigue from extended hospitalization
- Cognitive and psychological impacts, such as PTSD in long-term ICU patients
While ARDS cannot be completely prevented, managing its underlying causes can reduce risk. Prompt treatment of infections, injury, and lung-damaging conditions is essential.

Acute & Chronic Respiratory Failure
Respiratory failure occurs when the lungs cannot adequately exchange oxygen and carbon dioxide, resulting in dangerously low oxygen levels (hypoxemia) or high carbon dioxide levels (hypercapnia) in the blood. This condition can be acute (sudden onset) or chronic (long-term) and can affect various systems of the body due to the insufficient oxygen supply.
- Acute Respiratory Failure
This develops rapidly and often is a medical emergency that requires immediate intervention. It usually troubles for few minutes to hours.
- Chronic Respiratory Failure
This develops gradually over weeks, months or years and is often associated with complicated long term lung disorder or other chronic conditions.
- Hypoxemic Respiratory Failure
The primary issue is oxygen levels are low in the blood (PaO2 < 60 mmHg)
- Hypercapnic Respiratory Failure
The major problem is that carbon dioxide levels are often elevated due to inadequate ventilation.
- Acute Respiratory Distress Syndrome (ARDS)
- Pneumonia: Severe infections of the lungs can impair oxygen exchange.
- Pulmonary Embolism: Blood clots in the lungs block oxygen flow.
- Asthma or COPD Exacerbations: Sudden worsening of chronic conditions can cause acute respiratory failure.
- Trauma or Injury: Chest or lung injuries may lead to sudden respiratory failure.
- Drug Overdose: Opioids, sedatives, or other drugs can suppress breathing.
- Neuromuscular Disorders: Conditions like Guillain-Barre Syndrome can affect the muscles used for breathing.
- Severe shortness of breath (dyspnea)
- Rapid, shallow breathing
- Confusion, agitation, or anxiety
- Cyanosis (bluish color of lips, fingers, or skin)
- Rapid heart rate (tachycardia)
- Fatigue or lethargy
- Loss of consciousness in severe cases
- Oxygen Therapy: Administered to improve oxygen levels in cases of hypoxemic failure. High-flow nasal cannula or non-invasive ventilation may be used.
- Mechanical Ventilation: If oxygen therapy is insufficient, mechanical ventilation can assist or take over breathing. Intubation is often required for severe cases.
- Treat Underlying Causes: Antibiotics for infections, anticoagulants for pulmonary embolism, bronchodilators for asthma or COPD exacerbations, and corticosteroids for inflammation.
- Extracorporeal Membrane Oxygenation (ECMO): For severe cases where mechanical ventilation fails, ECMO may be used to oxygenate the blood outside the body.
Prognosis depends on the underlying cause and the rapidity of intervention. ARDS, for example, can lead to long-term lung damage even after recovery.
Advanced Respiratory Failure
This is a critical condition wherein the lungs no longer be able to provide sufficient oxygen to the bloodstream or remove carbon dioxide from the body with maximum medical support. Often it occurs as a progression from chronic respiratory failure or is severe outcome of acute respiratory failure that becomes unmanageable by conventional means. Also, such respiratory failures often require highly specialized care and life support systems.
- Hypoxemic Respiratory Failure: Severe deficiency in oxygen levels (PaO2 < 60 mmHg) that cannot be corrected even with high levels of supplemental oxygen.
- Hypercapnic Respiratory Failure: Excessive buildup of carbon dioxide (PaCO2 > 50 mmHg) due to inadequate ventilation, leading to respiratory acidosis.
- Combined Respiratory Failure: Both oxygen levels and carbon dioxide levels are critically abnormal, which often indicates widespread lung damage or failure in the body’s ability to regulate gas exchange.
- Chronic Obstructive Pulmonary Disease (COPD): In advanced stages, COPD may lead to irreversible lung damage that results in severe and persistent respiratory failure.
- End-Stage Interstitial Lung Disease: Scarring and fibrosis of lung tissue prevent effective gas exchange.
- End-Stage Cystic Fibrosis: Progressive mucus accumulation and infections lead to advanced respiratory complications.
- Advanced Pulmonary Hypertension: Increased pressure in the lungs’ arteries can result in severe strain on the lungs and heart, leading to failure.
- Neuromuscular Diseases: Progressive conditions like amyotrophic lateral sclerosis (ALS) or muscular dystrophy can eventually compromise the respiratory muscles.
- Severe ARDS: Acute Respiratory Distress Syndrome can escalate into advanced respiratory failure if the lungs are extensively damaged and cannot recover despite treatment.
- Advanced Obesity Hypoventilation Syndrome: Severe cases can lead to an inability to maintain adequate oxygen and carbon dioxide levels.
- End-Stage Heart Failure (Cor Pulmonale): Chronic right-sided heart failure due to lung diseases can worsen respiratory failure.
- Severe and persistent shortness of breath (dyspnea), even at rest
- Extreme fatigue and inability to perform even minimal physical activities
- Frequent exacerbations of lung conditions requiring hospitalization
- Cyanosis (bluish tint to the lips, skin, or fingernails) indicating dangerously low oxygen levels
- Morning headaches or confusion, often a sign of chronic carbon dioxide retention
- Rapid heart rate and high blood pressure, or conversely, low blood pressure in terminal stages
- Inability to speak in full sentences due to breathlessness
- Decreased consciousness or cognitive decline in severe hypercapnia
- Arterial Blood Gas (ABG): Regular monitoring of oxygen (PaO2) and carbon dioxide (PaCO2) levels is critical in diagnosing and managing advanced respiratory failure.
- Pulse Oximetry: Continuously used to monitor oxygen saturation levels but may be inadequate in severe cases.
- Chest X-ray or High-Resolution CT Scan: Helps identify extensive damage to the lung structure, such as fibrosis or emphysema.
- Pulmonary Function Tests (PFTs): Provides detailed assessment of lung function, typically showing severely reduced lung capacity in advanced cases.
- Right Heart Catheterization: Used to assess pulmonary hypertension, which is commonly associated with advanced respiratory failure.
- Oxygen and Ventilatory Support
- Long-term Oxygen Therapy (LTOT): Patients with advanced respiratory failure often require continuous oxygen supplementation at home or in a healthcare setting.
- Non-invasive Ventilation (NIV): Devices like CPAP or BiPAP can assist with breathing but may not be sufficient in advanced cases.
- Mechanical Ventilation: In more severe cases, mechanical ventilation may be necessary to take over or support the patient’s breathing. This can be invasive (intubation) or non-invasive in certain cases.
- Extracorporeal Membrane Oxygenation (ECMO): For patients with extreme respiratory failure, ECMO can oxygenate blood outside the body, serving as a last resort for those who fail to respond to conventional ventilation.
- Medications
- Bronchodilators: Used to open airways in patients with COPD or asthma, though their effect may be limited in advanced disease.
- Corticosteroids: Administered to reduce inflammation, particularly in conditions like severe COPD or ARDS.
- Diuretics: To manage fluid overload, particularly in patients with heart failure contributing to respiratory failure.
- Antibiotics: Used to treat or prevent frequent respiratory infections, which can exacerbate respiratory failure.
- Lung Transplantation
For eligible patients, lung transplantation may be considered in cases of advanced respiratory failure due to end-stage lung disease. This is typically reserved for patients with no other treatment options and a high chance of post-surgery survival.
- Palliative Care
In cases where curative treatment is no longer effective, palliative care focuses on providing relief from symptoms and improving the quality of life. This includes managing breathlessness, anxiety, and providing emotional support to patients and families.
Advanced respiratory failure often carries a poor prognosis, especially when caused by progressive or end-stage conditions. Long-term survival depends on the underlying cause, the success of treatments like ECMO or transplantation, and the patient’s overall health.
Complications
- Multi-organ failure due to severe hypoxemia and acidosis
- Pulmonary hypertension, leading to right-sided heart failure (cor pulmonale)
- Recurrent respiratory infections
- Muscle wasting and physical deconditioning due to prolonged illness and immobility
- Psychological impacts such as anxiety and depression from chronic breathlessness
- Early Treatment of Lung Diseases: Preventing the progression of chronic lung diseases through medications, lifestyle changes, and early intervention can delay the onset of advanced respiratory failure.
- Vaccinations: Influenza and pneumococcal vaccines reduce the risk of infections that can worsen respiratory conditions.
- Smoking Cessation: Quitting smoking is the most effective way to prevent the progression of diseases like COPD, which can lead to advanced respiratory failure.

Interventional Pulmonology
interventional pulmonology (IP) is a specialized field with pulmonary medicine and they majorly focus on invasive techniques to diagnose and treats condition that affects the lungs, pleura (lining of the lungs) and airways. This branch of medicine combines advanced endoscopic, imaging, and catheter-based procedures to manage complex respiratory diseases, offering alternatives to more invasive surgical options.
- Bronchoscopy: A flexible or rigid scope is passed through the nose or mouth into the lungs to examine the airways, take tissue samples (biopsies), or remove obstructions. It can be used for both diagnosis and therapy, both purposes.
- Endobronchial Ultrasound (EBUS): A minimally invasive procedure that combines bronchoscopy with ultrasound to visualize and biopsy lymph nodes or masses located near the airways, often used to diagnose lung cancer or other mediastinal diseases.
- Navigational Bronchoscopy: Uses advanced imaging technology (CT scans) to guide the bronchoscope to reach difficult areas of the lung, allowing for more precise biopsies of peripheral lung nodules.
- Thoracoscopy (Pleuroscopy): A minimally invasive procedure to examine and treat conditions of the pleura, such as pleural effusions (fluid buildup) or pleural disease. It is commonly used to diagnose pleural cancers and infections.
- Airway Stenting: Placement of stents in narrowed or obstructed airways caused by tumors, scarring, or other conditions to keep them open and improve breathing.
- Balloon Dilation (Bronchoplasty): Used to widen narrowed airways due to strictures, tumors, or scarring. This helps improve airflow in patients with severe airway obstructions.
- Pleurodesis: A procedure where the pleural space is intentionally scarred to prevent recurrent pleural effusions (fluid buildup around the lungs) in conditions like malignancy or chronic heart failure.
- Cryotherapy and Laser Therapy: Minimally invasive treatments used to remove or shrink tumors or abnormal tissue in the airways. Cryotherapy freezes the tissue, while laser therapy uses heat to destroy abnormal cells.
- Thoracentesis: A procedure to remove fluid from the pleural space around the lungs, commonly used in cases of pleural effusion to alleviate symptoms and diagnose the cause of fluid accumulation.
- Tracheobronchial Thermoplasty: A specialized treatment for severe asthma, where heat is applied to reduce the smooth muscle in the airways, decreasing their ability to constrict and improving airflow.
- Lung Cancer Diagnosis and Treatment: EBUS and navigational bronchoscopy are critical tools for diagnosing and staging lung cancer. Bronchoscopy can be used for tumor biopsy, while airway stenting and ablative therapies can relieve obstructions caused by tumors.
- Pleural Disease: Conditions like pleural effusion, pleural thickening, or mesothelioma may require thoracoscopy, thoracentesis, or pleurodesis to diagnose or manage the disease.
- Chronic Obstructive Pulmonary Disease (COPD): Interventional procedures like bronchoplasty or airway stenting can help patients with severe COPD who experience airway narrowing or obstruction.
- Asthma: In severe, treatment-resistant asthma, bronchial thermoplasty can reduce the frequency and severity of asthma attacks.
- Pulmonary Infections: Bronchoscopy may be used to obtain samples from the lungs for diagnosing difficult-to-treat lung infections such as tuberculosis or fungal infections.
- Airway Obstructions: Caused by tumors, foreign bodies, or scarring. Interventional pulmonology procedures such as stenting, balloon dilation, or cryotherapy can open blocked airways and improve breathing.
- Pneumothorax: Thoracoscopy and pleurodesis are often used to treat recurrent pneumothorax (collapsed lung) to prevent future episodes.
- Minimally Invasive: Procedures are performed using small incisions or natural body openings, which leads to faster recovery times, reduced pain, and shorter hospital stays compared to traditional surgery.
- Diagnostic Precision: Techniques like EBUS and navigational bronchoscopy allow for highly accurate biopsies, even from hard-to-reach lung regions, improving the diagnosis of lung cancers and other diseases.
- Therapeutic Relief: Interventional procedures can immediately relieve symptoms such as airway obstructions, pleural effusions, or bleeding, significantly improving a patient’s quality of life.
- Outpatient Procedures: Many interventional pulmonology procedures can be performed on an outpatient basis, meaning the patient can go home the same day, which is less disruptive and more cost-effective.
Though interventional pulmonology procedures are generally safe, they do carry some risks, such as:
- Bleeding or infection at the procedure site
- Pneumothorax (collapsed lung) following a bronchoscopy or thoracentesis
- Airway perforation or damage during bronchoscopy
- Reaction to sedation or anesthesia
- Fluid leakage in pleurodesis or thoracoscopy
- Respiratory failure in patients with severely compromised lung function
Dio Bronchoscopy
Dio bronchoscopy refers to an advanced technique in bronchoscopy that combines both diagnostic and interventional capabilities in a single procedure. It uses specialized equipment to not only visualize the airways and lung structures but also perform various interventions during the same procedure. Dio bronchoscopy is designed to enhance the precision of diagnosis and treatment, reducing the need for multiple procedures. It is especially useful in managing complex pulmonary diseases such as lung cancer, infections, and airway obstructions.
By integrating advanced tools like endobronchial ultrasound (EBUS), high-definition imaging, and therapeutic modalities, Dio bronchoscopy offers comprehensive solutions for both diagnosis and treatment of lung and airway diseases.
- Combined Diagnostic and Therapeutic Approach: The key feature of Dio bronchoscopy is the ability to diagnose and treat lung conditions simultaneously. For example, if a suspicious lesion is found during bronchoscopy, a biopsy can be taken immediately, and certain treatments, like tumor debulking or airway stenting, can be performed in the same session.
- High-Resolution Imaging: Dio bronchoscopy systems are equipped with state-of-the-art imaging technology, providing high-definition views of the airways and lung tissues, enhancing the accuracy of diagnosis.
- Endobronchial Ultrasound (EBUS): An essential tool integrated into Dio bronchoscopy, EBUS allows for real-time imaging of the lymph nodes and masses adjacent to the airways, improving diagnostic accuracy for lung cancer and other mediastinal diseases.
- Advanced Navigation Systems: Dio bronchoscopy utilizes advanced computer-assisted navigation systems to guide the bronchoscope precisely to areas of interest, such as small peripheral lung nodules or difficult-to-reach areas, which may not be accessible by standard bronchoscopy techniques.
- Comprehensive Care: Dio bronchoscopy allows for both diagnosis and treatment in one session, minimizing the need for additional invasive procedures or surgeries, which is especially beneficial for critically ill or high-risk patients.
- High Diagnostic Accuracy: The combination of high-definition imaging, EBUS, and navigational technologies increases the accuracy of diagnosis, particularly for small or difficult-to-reach lesions.
- Minimally Invasive: As a minimally invasive procedure, Dio bronchoscopy reduces recovery time, the risk of complications, and the need for hospital stays compared to traditional surgical methods.
- Immediate Symptom Relief: Procedures like airway stenting or tumor removal can provide quick relief from symptoms such as shortness of breath, allowing patients to breathe more easily.
- Outpatient Procedure: Many Dio bronchoscopy procedures can be performed on an outpatient basis, allowing patients to return home the same day and resume normal activities sooner.
- Biopsies: Tissue samples can be taken from suspicious masses or nodules during the procedure. These biopsies help in diagnosing lung cancer, infections, or inflammatory diseases. Dio bronchoscopy allows precise targeting of even small, difficult-to-access lesions.
- Endobronchial Ultrasound-Guided Biopsy (EBUS-TBNA): Dio bronchoscopy uses EBUS to guide the biopsy needle to lymph nodes or masses near the bronchi. This is especially useful for diagnosing and staging lung cancer, as it allows for sampling of lymph nodes without the need for more invasive surgery.
- Airway Stenting: If the airways are narrowed or blocked due to tumors, scar tissue, or other obstructions, Dio bronchoscopy can place stents to keep the airway open, improving breathing and relieving symptoms.
- Tumor Ablation: Dio bronchoscopy can be used for removing or shrinking tumors using techniques such as cryotherapy (freezing) or laser therapy, providing immediate symptom relief for patients with airway obstruction due to cancer.
- Bronchial Thermoplasty: This specialized procedure, offered as part of Dio bronchoscopy for patients with severe asthma, involves delivering heat to reduce the amount of smooth muscle in the airways, leading to better airflow and reduced asthma symptoms.
- Foreign Body Removal: Dio bronchoscopy is also highly effective in removing foreign objects or mucus plugs that may be blocking the airways, especially in emergency situations or pediatric cases.
- Pleuroscopy/Thoracoscopy Assistance: Dio bronchoscopy may also assist in pleuroscopy, where the lining of the lungs is examined or biopsied for diagnosing pleural diseases like mesothelioma or recurrent pleural effusion.
- Lung Cancer Diagnosis and Staging: Dio bronchoscopy is widely used to diagnose lung cancer by obtaining tissue samples from the lungs or mediastinal lymph nodes, and it helps in determining the stage of cancer, which is critical for treatment planning.
- Obstructive Lung Diseases: Conditions like chronic obstructive pulmonary disease (COPD) or asthma, where airway obstruction is a problem, can benefit from stenting, tumor removal, or bronchial thermoplasty.
- Pulmonary Infections: Dio bronchoscopy is useful in diagnosing lung infections, especially when the causative organism is difficult to identify through non-invasive methods. It can also be used to drain abscesses or remove infected tissue.
- Airway Obstructions: Tumors, foreign bodies, or inflammatory conditions causing narrowing or blockage of the airways can be treated using Dio bronchoscopy techniques such as stenting or tumor ablation.
- Lung Nodules: Small lung nodules identified on imaging scans (e.g., CT scans) can be precisely biopsied using navigational bronchoscopy, improving the diagnosis of early-stage lung cancer or other pulmonary conditions.
Patients undergoing Dio bronchoscopy will need to fast for a few hours before the procedure and may be advised to stop certain medications (e.g., blood thinners). Sedation or general anesthesia is typically used to ensure patient comfort during the procedure. After the procedure, patients will be monitored until the effects of sedation wear off, and in most cases, they can return home the same day.
Autofluorescence-Assisted Bronchoscopy (AAL)
Autofluorescence-Assisted Bronchoscopy or AAL, is an advanced diagnostic technique used to detect early-stage lung cancer and other abnormal changes in the airways that may not be visible with traditional bronchoscopy. The process includes the utilization of autofluorescence imaging, where the tissues in the airways are illuminated with a specific type of light, causing them to emit fluorescence. Healthy tissues and abnormal tissues emit different wavelengths of light, allowing for the detection of pre- cancerous or cancerous lesions in the bronchi and lungs.
This technology is valuable for identifying lesions at an early stage, especially when they are small or located in the superficial layers of the airways. Early detection through AAL can lead to better outcomes in the treatment of lung cancer and other respiratory diseases.
- Increased Sensitivity for Early Lesions: AAL has a higher sensitivity than standard white-light bronchoscopy for detecting early and subtle changes in the airway epithelium. This makes it particularly valuable in finding pre-cancerous or early-stage cancerous lesions that would otherwise go unnoticed.
- Real-Time Imaging: The procedure allows the physician to take immediate and real-time images of the airways. This makes it easy to access the abnormal areas and take necessary actions such as starting treatment or the need to perform biopsy.
- Minimally Invasive: Like traditional bronchoscopy, AAL is minimally invasive and can often be performed on an outpatient basis, i.e. patients can return home the same day.
- Improved Lung Cancer Outcomes: By detecting cancer early, when it is most treatable, AAL has the potential to improve survival rates for patients with lung cancer.
- Early Cancer Detection: AAL is primarily used to detect early-stage lung cancers or pre-cancerous changes in patients at high risk for lung cancer, such as smokers or those with a history of chronic obstructive pulmonary disease (COPD) or previous cancer.
- Biopsies: If suspicious areas are identified through autofluorescence, targeted biopsies can be performed immediately during the procedure. The autofluorescence highlights abnormal tissue, allowing the physician to precisely locate and sample the affected areas.
- Monitoring for Recurrence: Patients who have previously undergone treatment for lung cancer may undergo AAL to monitor for recurrence. This technique helps in detecting abnormal changes in the airways that may indicates return of cancer.
- Follow-Up in High-Risk Patients: AAL is also useful for patients with a high risk of developing lung cancer, such as long-term smokers or those with occupational exposures (e.g., asbestos). Regular screenings with AAL can help in identifying early changes that warrant closer monitoring or treatment.
- Autofluorescence Imaging: AAL uses blue or violet light to stimulate the natural fluorescence of the tissues lining the airways. Normal tissues produce a greenish autofluorescence, while abnormal or cancerous tissues appear darker, typically in shades of brown or red. This contrast allows to identify the areas that might be missed using standard white-light bronchoscopy.
- Early Detection of Cancer: AAL is highly effective in detecting early-stage lung cancer or pre-cancerous lesions that are still confined to the bronchial mucosa, enabling earlier intervention and potentially improving patient survival rates.
- Adjunct to Standard Bronchoscopy: AAL is typically used in conjunction with conventional white-light bronchoscopy. The combination of both imaging techniques increases the diagnostic accuracy, especially for detecting small or early-stage lesions.
- Non-invasive Visualization: Like standard bronchoscopy, AAL is a minimally invasive procedure that provides real-time imaging of the airways without the need for surgical exploration.
- Lung Cancer Screening: AAL is particularly useful in screening individuals at high risk for lung cancer, such as heavy smokers or those with a family history of lung cancer. Its ability to detect pre-cancerous changes offers a critical advantage in early diagnosis.
- Surveillance of Pre-Cancerous Lesions: Patients with known pre-cancerous conditions, such as dysplasia or carcinoma in situ, can be monitored with AAL for progression or transformation into invasive cancer.
- Assessing Lesions Found on Imaging: If abnormal areas are identified on imaging studies like CT scans but are difficult to visualize through conventional bronchoscopy, AAL can help in more accurately locating and diagnosing these lesions.
- Evaluating Unexplained Symptoms: In patients with unexplained respiratory symptoms such as persistent cough, blood in the sputum (hemoptysis), or recurrent infections, AAL may help detect abnormalities that are not visible using standard techniques.
- Before undergoing AAL, patients may be asked to refrain from eating or drinking for several hours.
- Medications that affect blood clotting, such as blood thinners, may need to be stopped temporarily.
- The procedure is usually performed under conscious sedation, meaning the patient will be awake but relaxed and pain-free during the bronchoscopy.
After the procedure, patients are monitored for any complications, and most can go home the same day. Some minor side effects, such as a sore throat or cough, may occur but usually resolve within a few days.
While AAL is generally safe, it does carry some risks, similar to those of standard bronchoscopy, including:
- Bleeding: There is a small risk of bleeding, particularly if a biopsy is performed.
- Infection: In rare cases, infection can occur following the procedure.
- Pneumothorax: A small risk of lung collapse exists, especially if the bronchoscopy involves taking tissue samples from deeper areas of the lungs.
- Sedation-Related Complications: As with any procedure that involves sedation, there is a small risk of adverse reactions to the sedative or anesthesia.
Endobronchial Ultrasound (EBUS) - TB
Endobronchial Ultrasound (EBUS) – Transbronchial Biopsy (TB) is a specialized procedure that combines bronchoscopy with ultrasound to obtain real-time imaging and perform biopsies from areas surrounding the bronchi, particularly the lymph nodes and masses in the mediastinum (the central chest area). This technique is primarily used to diagnose the stage of lung cancer, as well as to evaluate infections like tuberculosis (TB) and other diseases involving the lymph nodes or lungs.
EBUS-TB provides a minimally invasive way to access areas that were previously only reachable through more invasive surgeries, such as mediastinoscopy. It allows doctors to collect tissue samples (biopsies) from the lymph nodes and other areas for diagnosis, with the aid of ultrasound guidance, ensuring precise and safe sampling.
- High Diagnostic Accuracy: EBUS-TB allows for precise targeting of abnormal lymph nodes and masses, leading to a high diagnostic accuracy, particularly for lung cancer and other thoracic diseases.
- Minimally Invasive: Compared to surgical alternatives like mediastinoscopy, EBUS-TB is minimally invasive, resulting in less pain, quicker recovery, and fewer complications.
- Outpatient Procedure: Most EBUS-TB procedures are performed on an outpatient basis, meaning patients can return home the same day with minimal downtime.
- Real-Time Imaging: The real-time ultrasound imaging ensures that the biopsy needle is accurately placed, reducing the risk of complications and increasing the likelihood of obtaining a good tissue sample.
- Safe and Effective: EBUS-TB has a lower risk of complications compared to more invasive surgical procedures, and it is considered safe even for patients with compromised health or other medical conditions.
- Lymph Node Biopsy: One of the primary uses of EBUS-TB is to obtain samples from enlarged lymph nodes near the trachea and bronchi. This is particularly important in diagnosing and staging lung cancer, as cancer often spreads to these lymph nodes before moving to other parts of the body.
- Transbronchial Needle Aspiration (TBNA): Using a fine needle, the physician can collect samples from masses or lymph nodes located near the airways. This procedure is often used to diagnose cancer, tuberculosis, lymphoma, or infections that cause lymph node enlargement.
- Diagnosis of Tuberculosis (TB): EBUS-TB is valuable for diagnosing TB in cases where the disease involves the lymph nodes or mediastinum. The procedure helps in obtaining samples from infected nodes that can be tested for TB bacteria, allowing for a definitive diagnosis.
- Staging of Lung Cancer: EBUS-TB is critical in determining the stage of lung cancer, which helps in treatment planning. By identifying whether cancer has spread to the lymph nodes or surrounding tissues, the procedure provides essential information for assessing the prognosis and treatment options.
- Evaluation of Other Lung Diseases: EBUS-TB can be used to investigate other conditions that cause enlarged lymph nodes, such as sarcoidosis, lymphoma, and fungal infections. The tissue samples collected during the procedure can help in making an accurate diagnosis.
- Ultrasound-Guided Imaging: The key advantage of EBUS is its ability to provide real-time ultrasound images of the structures surrounding the bronchi. This includes lymph nodes, blood vessels, and masses, which are difficult to visualize using standard bronchoscopy.
- Minimally Invasive: EBUS-TB is a less invasive alternative to surgical procedures like mediastinoscopy. It is performed through the mouth or nose, using a flexible bronchoscope equipped with an ultrasound probe and a fine needle for biopsy.
- Precise Biopsy: With ultrasound guidance, EBUS allows the physician to accurately target lymph nodes and masses, ensuring that the biopsies are taken from the correct area, which is crucial for accurate diagnosis.
- Diagnostic and Staging Tool: EBUS-TB is most commonly used for diagnosing and staging lung cancer, but it is also valuable in diagnosing other diseases that affect the lungs and mediastinal lymph nodes, such as tuberculosis (TB) and sarcoidosis.
- Before the procedure, patients are usually advised to fast for several hours.
- Medicines that affect blood clotting such as anticoagulants are stopped on a temporary basis.
- The procedure is typically performed under general anesthesia and sedation to provide patient comfort.
- Once the procedure is completed, patients are monitored in a recovery area for swelling and if everything is fine they’re released to go home. Normal activities can be resumed within a day or two.
Although EBUS-TB is generally safe, it does carry some risks, including:
- Bleeding: There is a small risk of bleeding at the biopsy site, especially if the lymph nodes or masses are located near blood vessels.
- Pneumothorax (Collapsed Lung): This is a rare complication but can occur if the biopsy needle accidentally punctures the lung.
- Infection: Any procedure that involves penetrating the body’s tissues carries a risk of infection, though this is rare with EBUS-TB.
- Reactions to Sedation: Since the procedure is often performed under sedation, there is a small risk of adverse reactions to the sedative or anesthetic agents.

Therapeutic Bronchoscopy
Therapeutic bronchoscopy is an invasive procedure used to treat various conditions that affect the airways and lungs. Unlike diagnostic bronchoscopy, which is used to diagnose lung diseases by visualizing the airways and collecting samples, therapeutic bronchoscopy is specifically employed to directly address underlying conditions. These interventions include removing airway obstructions, treating tumors, managing conditions that impair airflow, and controlling bleeding.
Therapeutic bronchoscopy is performed using a bronchoscope—a thin, flexible tube equipped with a camera and specialized instruments that enable the treatment of various airway conditions without the need for surgical intervention. Depending on the specific treatment required, bronchoscopy can be performed under local anesthesia with sedation or general anesthesia.
- Minimally Invasive: Therapeutic bronchoscopy provides a less invasive option compared to open surgery, resulting in less pain, faster recovery, and fewer complications.
- Real-Time Treatment: The ability to directly visualize and treat problems within the airway during the procedure makes therapeutic bronchoscopy highly effective for managing airway obstructions, bleeding, and other conditions.
- Immediate Symptom Relief: Many patients experience immediate relief from symptoms such as shortness of breath or bleeding after therapeutic bronchoscopy interventions, particularly in cases of airway obstructions.
- Outpatient Procedure: Most therapeutic bronchoscopies are performed on an outpatient basis or with a short hospital stay, minimizing the need for extended hospitalization and allowing for faster return to normal activities.
- Versatility in Treating Multiple Conditions: Therapeutic bronchoscopy can be used to treat a wide range of airway and lung conditions, from cancer-related obstructions to benign strictures and foreign body removal, making it a versatile tool in pulmonary medicine.
- Non-Surgical Airway Management: Therapeutic bronchoscopy offers a non-surgical approach to treat airway and lung diseases, providing real-time visualization and direct intervention without the need for traditional open surgery.
- Precision and Minimally Invasive: The procedure allows doctors to precisely target problem areas in the airways or lungs, such as obstructions, tumors, or bleeding sites, using minimally invasive techniques.
- Real-Time Intervention: With the aid of bronchoscope imaging, doctors can immediately perform therapeutic interventions such as removing foreign objects, placing stents, or reducing airway obstructions caused by tumors.
- Outpatient or Short Hospital Stay: Most therapeutic bronchoscopy procedures are performed on an outpatient basis, allowing for quicker recovery and minimal downtime.
- Airway Stenting: Bronchoscopy is often used to place stents in the airways to keep them open in cases of obstruction caused by tumors, strictures (narrowing of the airway), or external compression from masses. Stents help restore airflow and improve breathing in patients with airway blockages.
- Tumor Debulking: In patients with lung cancer or benign tumors that are obstructing the airway, therapeutic bronchoscopy can be used to reduce the size of the tumor (debulking) using techniques like laser therapy, cryotherapy (freezing), or electrocautery (heat). This helps improve breathing and alleviate symptoms.
- Foreign Body Removal: Therapeutic bronchoscopy is commonly used to remove foreign objects that may have been accidentally inhaled into the airways, especially in children or adults with impaired swallowing. The bronchoscope allows for the safe retrieval of these objects without the need for surgery.
- Management of Airway Bleeding (Hemoptysis): Bronchoscopy can be used to control bleeding in the airways (hemoptysis) by identifying the source of bleeding and applying techniques such as cauterization, laser therapy, or placing a balloon to stop the bleeding.
- Bronchial Thermoplasty: This specialized form of therapeutic bronchoscopy is used to treat severe, uncontrolled asthma. The procedure involves applying controlled heat to the airway walls to reduce the smooth muscle mass, helping to prevent airway constriction and reduce asthma attacks.
- Balloon Dilation: For patients with airway narrowing (stenosis) due to scarring or inflammation, therapeutic bronchoscopy can be used to dilate (widen) the airway using a balloon catheter. This improves airflow and relieves symptoms of airway obstruction.
- Malignant Airway Obstruction: Therapeutic bronchoscopy is frequently used to treat airway obstructions caused by lung cancer or tumors that compress or invade the airways. Techniques like tumor debulking, stenting, or laser therapy help improve breathing and quality of life.
- Benign Airway Obstruction: Conditions such as tracheal or bronchial stenosis (narrowing) due to scarring, trauma, or inflammation can be treated with balloon dilation or stent placement to restore airflow.
- Foreign Body Aspiration: In cases where a foreign object has been inhaled into the airways, therapeutic bronchoscopy provides a safe and effective way to remove the object without surgery.
- Severe Asthma: Bronchial thermoplasty is used as a treatment for severe asthma that does not respond to conventional therapies. It helps reduce the frequency and severity of asthma attacks by decreasing the airway’s ability to constrict.
- Hemoptysis (Airway Bleeding): Bronchoscopy is used to control significant airway bleeding by identifying the bleeding site and applying therapeutic measures to stop it, such as cauterization or balloon tamponade.
- Airway Strictures: Patients with airway strictures due to chronic inflammation, infection, or trauma may benefit from therapeutic bronchoscopy procedures like balloon dilation or stent placement to relieve the obstruction.
While therapeutic bronchoscopy is generally safe, there are some risks associated with the procedure, including:
- Bleeding: There is a risk of bleeding, especially when treating tumors or controlling hemoptysis.
- Pneumothorax (Collapsed Lung): In rare cases, therapeutic interventions can lead to a pneumothorax, where air escapes into the space around the lungs, requiring treatment with a chest tube.
- Infection: Any invasive procedure carries a small risk of infection, though this is rare in bronchoscopy.
- Airway Trauma: Manipulation of the airways during bronchoscopy can cause minor trauma, such as irritation or swelling, though serious complications are uncommon.
Tumor Debulking
Tumor debulking is a therapeutic procedure used to reduce the size of a tumor that is causing airway obstruction or impeding respiratory function. This intervention is typically performed through bronchoscopy, a minimally invasive technique where specialized instruments are used to remove or reduce tumor mass from the airway or bronchial tree. Tumor debulking is not intended to cure the underlying cancer but to relieve symptoms like shortness of breath, wheezing, or coughing caused by the tumor pressing on the airways.
This procedure is often utilized in cases where tumors are inoperable due to their size, location, or the patient’s general condition. It may also be used in palliative care to improve quality of life by alleviating obstructive symptoms. Tumor debulking can be performed using various techniques such as laser therapy, electrocautery, cryotherapy, or mechanical removal, depending on the location and nature of the tumor.
- Improved Breathing and Symptom Relief: By reducing the size of the tumor and clearing the airway, patients often experience immediate improvement in their breathing and a reduction in symptoms such as coughing and wheezing.
- Minimally Invasive: Tumor debulking is generally performed using bronchoscopy, which is less invasive than open surgery. This leads to a faster recovery, less postoperative pain, and fewer complications.
- Palliative Care Option: For patients with advanced cancer or those who are not candidates for surgery, tumor debulking offers an effective way to improve quality of life by reducing airway obstruction and improving respiratory function.
- Outpatient or Short Hospital Stay: Most tumor debulking procedures are performed on an outpatient basis or with a short hospital stay, minimizing disruption to the patient’s daily life.
- Symptom Relief: Tumor debulking helps to relieve symptoms caused by tumor-induced airway obstruction, such as shortness of breath, coughing, and wheezing, thus improving respiratory function.
- Minimally Invasive: The procedure is usually performed through bronchoscopy, which avoids the need for open surgery, leading to quicker recovery times and fewer complications.
- Palliative Intent: While it does not cure cancer, tumor debulking is a palliative procedure aimed at improving quality of life by removing or reducing the tumor mass that obstructs airflow in the lungs.
- Variety of Techniques: Depending on the tumor’s characteristics and the patient’s condition, a variety of techniques can be used for debulking, including laser ablation, cryotherapy (freezing), and electrocautery (heat).
- Laser Therapy: Laser therapy uses focused light energy to vaporize or cut away tumor tissue, reducing its size and clearing the airway. It is particularly useful for endobronchial tumors that block the main bronchi or trachea.
- Electrocautery: Electrocautery involves using heat generated by electric currents to burn away tumor tissue. This technique is effective in shrinking tumors and stopping any bleeding that may result from the procedure.
- Cryotherapy: In this technique, extreme cold is applied to freeze and destroy tumor cells. Cryotherapy is often used for tumors that are located deeper within the airway or for recurrent tumors that cannot be completely removed by other methods.
- Mechanical Tumor Removal: In some cases, mechanical tools such as forceps or snares are used to physically remove parts of the tumor. This is typically done when the tumor is soft and easily accessible through bronchoscopy.
- Airway Obstruction: Tumor debulking is primarily indicated for patients whose airways are obstructed by tumors, causing breathing difficulties. This is common in patients with lung cancer, bronchial tumors, or metastases that invade the airways.
- Inoperable Tumors: When tumors cannot be surgically removed due to their size, location, or the patient’s inability to tolerate surgery, tumor debulking via bronchoscopy offers a less invasive alternative.
- Palliative Care: For patients with advanced or metastatic cancer, tumor debulking is used as a palliative measure to improve respiratory function and alleviate symptoms, rather than attempting to cure the cancer.
- Recurrent Tumor Growth: Tumor debulking may be used for recurrent tumor growth in the airway following initial treatment. It helps manage symptoms and prevent airway obstruction.
Before undergoing tumor debulking, patients typically undergo a series of evaluations, including imaging studies (CT scans, MRIs, or PET scans) to assess the tumor’s size and location. The procedure is usually performed under sedation or general anesthesia, depending on the patient’s condition and the complexity of the tumor. Patients may be asked to fast for several hours before the procedure.
After the procedure, patients are monitored in a recovery area. Depending on the complexity of the debulking and the patient’s overall condition, they may be discharged the same day or require a short hospital stay for observation. Follow-up care includes monitoring for complications such as bleeding or infection, and additional treatments such as radiation or chemotherapy may be planned.
Although tumor debulking is a relatively safe procedure, there are potential risks and complications, including:
- Bleeding: Tumor debulking can cause bleeding, especially if the tumor is highly vascular. Electrocautery or laser techniques are often used to control bleeding.
- Pneumothorax (Collapsed Lung): There is a small risk of pneumothorax if air escapes into the pleural space during the procedure, although this is rare and usually managed with a chest tube.
- Infection: As with any invasive procedure, there is a risk of infection at the site where the bronchoscope is inserted or within the lungs.
- Tumor Recurrence: While debulking can reduce the tumor size, it does not cure cancer, and tumor regrowth or recurrence is possible.

Airway stenosis
Airway stenosis refers to the narrowing of the airway that occurs in the trachea, bronchi, or larynx. This condition reduces airflow, causing breathing problems and symptoms such as stridor (a high-pitched wheezing sound), shortness of breath, or recurrent respiratory infections. Airway stenosis can result from various factors, including trauma, infections, prolonged intubation, inflammatory diseases, or tumors. It is classified as either congenital (present at birth) or acquired (developed later in life).
The severity of this condition varies from mild to life-threatening, depending on the degree of narrowing and the underlying cause. In severe cases, airway stenosis can completely block airflow and may require medical intervention.
- Congenital Airway Stenosis:
This type of abnormality occurs due to issues in fetal development and may present symptoms early in life. Children with congenital stenosis are often associated with conditions such as vascular rings or congenital malformations.
- Acquired Airway Stenosis:
This more common form of stenosis usually occurs after prolonged intubation, infections such as tuberculosis, autoimmune diseases such as granulomatosis with polyangiitis (formerly Wegener’s), radiation therapy, or neck trauma.
- Subglottic Stenosis:
This specific type of stenosis occurs below the vocal cords and can be either congenital or acquired. It is a common area affected by prolonged intubation.
Airway stenosis is diagnosed through a combination of clinical evaluation, imaging studies, and endoscopic examinations.
- Clinical Evaluation: Patients with airway stenosis often present with breathing difficulties, stridor, or recurrent respiratory infections. Physicians will assess the patient’s history, including any history of trauma, surgery, or intubation.
- Imaging Studies:
- CT Scan: A computed tomography (CT) scan can provide detailed images of the airway and help determine the location and extent of the stenosis.
- MRI: Magnetic resonance imaging (MRI) may also be used in certain cases to provide a clearer picture of soft tissues around the airway.
- Bronchoscopy: The most definitive diagnostic tool for airway stenosis is bronchoscopy. This procedure involves inserting a bronchoscope (a thin, flexible tube with a camera) into the airway to directly visualize the stenosis, assess its severity, and, in some cases, collect biopsy samples.
- Pulmonary Function Tests (PFTs): These tests measure how well the lungs are functioning and can help quantify the degree of airflow obstruction caused by the stenosis.
The treatment of airway stenosis depends on the severity of the narrowing and the underlying cause. Common treatment options include:
- Balloon Dilation: A minimally invasive procedure in which a balloon is inserted through a bronchoscope and inflated at the site of the stenosis to stretch and widen the narrowed airway.
- Laser Ablation: This technique involves using laser energy to cut away the scar tissue or abnormal growth causing the stenosis. Laser therapy is often combined with other interventions like balloon dilation.
- Stent Placement: In cases of recurrent or severe stenosis, a stent (a small, expandable tube) may be placed in the airway to keep it open. Stents can be either temporary or permanent, depending on the cause of the stenosis.
- Surgical Resection: In more severe cases, surgical resection may be necessary to remove the affected portion of the airway, followed by reconstruction to restore normal airflow. This is typically reserved for patients who do not respond to less invasive treatments.
- Medical Management: In cases where stenosis is caused by inflammation or autoimmune disease, medical treatments such as corticosteroids or immunosuppressive drugs may be used to reduce inflammation and prevent further narrowing.
Prognosis
- Mild to Moderate Stenosis: With appropriate treatment such as balloon dilation or laser therapy, many patients experience long-term relief of symptoms. Regular follow-up is required to monitor for recurrence.
- Severe Stenosis: Surgical intervention can provide effective long-term relief, but complications such as recurrent stenosis or infection can occur. Prognosis is more guarded in these cases, especially if the underlying cause (such as cancer or autoimmune disease) persists.
- Recurrent Stenosis: Some patients may require repeated interventions, particularly if they have a history of prolonged intubation, tracheal injury, or other risk factors.
Complications
- Airway Obstruction: If untreated, severe airway stenosis can lead to life-threatening airway obstruction, requiring emergency intervention.
- Infection: Surgical interventions or stent placement carry a risk of infection, which may require antibiotic treatment.
- Tracheoesophageal Fistula: Rarely, aggressive surgical or endoscopic procedures can result in the formation of a fistula (an abnormal connection) between the trachea and the esophagus, which can cause serious complications like aspiration.
- Recurrence: Even after successful treatment, airway stenosis can recur, particularly if the underlying cause (e.g., scarring or inflammation) is not adequately controlled.
Preventing airway stenosis largely focuses on minimizing the risk factors that contribute to its development. Key strategies include:
- Careful Management of Intubation: Prolonged intubation is a major cause of acquired airway stenosis. Using smaller-sized tubes and reducing the duration of intubation when possible can help prevent trauma to the airway.
- Proper Postoperative Care: In patients who undergo neck or thoracic surgeries, appropriate postoperative care, including airway monitoring, can help prevent scarring and the development of stenosis.
- Prompt Treatment of Infections: Early treatment of respiratory infections, such as tuberculosis or fungal infections, can reduce the risk of scarring and airway damage that may lead to stenosis.
- Management of Inflammatory Diseases: Patients with autoimmune or inflammatory diseases that affect the airways (e.g., granulomatosis with polyangiitis or sarcoidosis) should be managed with appropriate medical therapy to prevent airway damage and stenosis.
- Avoiding Tracheal Injury: Patients with a history of trauma or surgery involving the airway should be carefully monitored for signs of stenosis, and any injury should be managed promptly to minimize long-term damage.
Airway Spigot
An airway spigot, also known as an endobronchial blocker, is a medical device used in the field of pulmonology to temporarily block or occlude a segment of the bronchial tree. These spigots are primarily used in cases where there is air leakage (such as in bronchopleural fistula), persistent airways bleeding (hemoptysis), or when isolating a diseased portion of the lung is necessary to prevent further complications. The spigot is designed to fit snugly into the airway, effectively sealing off the section and allowing for healing or preventing further damage to healthy lung tissue.
Airway spigots are often used in the management of complex pulmonary conditions where traditional surgical interventions may be too risky or unnecessary. Their application can be a temporary measure or part of a longer-term management strategy, depending on the underlying cause of the airway problem.
- Bronchopleural Fistula (BPF): One of the primary uses of an airway spigot is to seal bronchopleural fistulas, which are abnormal connections between the bronchial tree and the pleural space (the space surrounding the lungs). BPFs often occur after lung surgery or in cases of severe lung infections and can lead to life-threatening air leaks.
- Hemoptysis (Airway Bleeding): Spigots are also used to control life-threatening airway bleeding (hemoptysis), which can arise from a variety of conditions, such as tuberculosis, cancer, or severe bronchiectasis. In these cases, blocking the bleeding airway segment helps control the hemorrhage until definitive treatment is applied.
- Isolating Diseased Lung Segments: In certain lung infections or when a localized disease is present, an airway spigot can be used to isolate a specific lung segment, preventing further spread of infection or complications such as aspiration.
The need for an airway spigot is typically determined after a thorough diagnostic workup that includes the following:
- Bronchoscopy: This procedure is used to directly visualize the airways and assess the area that requires isolation or occlusion. It helps identify the presence of fistulas, bleeding points, or infection.
- Imaging Studies:
- CT Scan: A CT scan provides detailed images of the lungs and airways, helping to locate fistulas, infections, or areas of collapsed lung. It is an essential tool for planning the placement of the spigot.
- Chest X-ray: Often used to assess the general condition of the lungs and detect any abnormal air accumulation, which could indicate a bronchopleural fistula.
- Pulmonary Function Tests (PFTs): These tests help determine the overall lung function and assess whether the patient can tolerate the occlusion of a lung segment.
- Blood Gas Analysis: In cases of respiratory distress, blood gas analysis is used to measure oxygen and carbon dioxide levels, ensuring that occluding a segment of the lung will not severely impact breathing.
The airway spigot is placed via a bronchoscopy, a minimally invasive procedure performed under sedation or general anesthesia. The treatment plan includes the following steps:
- Bronchoscopic Placement:
- A flexible bronchoscope is inserted into the airway, and the spigot is guided into position. The spigot is sized appropriately to fit snugly within the targeted airway segment, creating a seal that blocks airflow or fluid.
- Depending on the location and size of the airway needing occlusion, different types and sizes of spigots can be used, such as the Watanabe spigot or silicon-based devices.
- Air Leak Management: In cases of bronchopleural fistula, the spigot effectively seals the air leak, allowing the pleural space to heal and preventing further air escape.
- Control of Hemoptysis: When used for severe airway bleeding, the spigot blocks the affected bronchus, stopping blood from entering the airway and preventing aspiration into healthy lung segments.
- Monitoring and Adjustment: After placement, the spigot may be monitored via imaging studies or repeat bronchoscopies to ensure correct positioning. In some cases, the spigot can be removed after a certain period once the underlying condition (such as a healed fistula) has resolved.
The prognosis for patients treated with an airway spigot depends on the underlying condition being managed. For example:
- Bronchopleural Fistula: Airway spigot placement can result in the complete healing of the fistula in many cases, especially if the underlying cause (e.g., infection, surgery) is addressed.
- Hemoptysis: Patients with massive hemoptysis may experience temporary relief and stabilization until more definitive treatments, such as surgery or embolization, can be performed.
- Localized Lung Disease: Isolating diseased lung segments with a spigot can prevent further damage or complications, improving overall lung function once the infection or disease has resolved.
Complications
While airway spigots are generally safe and effective, there are potential complications associated with their use, including:
- Migration or Displacement: The spigot may shift from its intended position, requiring a repeat bronchoscopy to reposition or remove it.
- Airway Obstruction: If the spigot inadvertently blocks too large a portion of the airway, it can lead to respiratory distress or atelectasis (collapse of lung tissue). Careful sizing and placement reduce this risk.
- Infection: Although rare, there is a risk of secondary infection at the site of the spigot. This can be managed with antibiotics and close monitoring.
- Granulation Tissue Formation: Over time, the body may react to the presence of the spigot by forming granulation tissue (scar tissue), which can lead to further airway narrowing. In such cases, removal or replacement of the spigot may be necessary.
- Limited Ventilation: In some cases, blocking a portion of the airway can lead to reduced lung ventilation, causing temporary hypoxia or respiratory compromise.
Preventing the need for an airway spigot typically involves reducing the risk factors for conditions that may lead to airway complications, such as bronchopleural fistulas or massive hemoptysis. Key preventive strategies include:
- Minimizing Surgical Risks: Careful surgical techniques during lung surgeries, such as pneumonectomy or lobectomy, can reduce the risk of developing bronchopleural fistulas.
- Infection Control: Prompt treatment of pulmonary infections such as tuberculosis or fungal infections can prevent damage to the lung tissue and reduce the likelihood of fistula formation or airway bleeding.
- Trauma Prevention: In patients with a history of trauma to the chest or airways, close monitoring for complications like airway leaks or fistulas can prevent the need for spigot placement.
- Careful Management of Lung Diseases: For patients with underlying conditions like bronchiectasis or chronic obstructive pulmonary disease (COPD), careful management and regular monitoring can help prevent airway damage that might require a spigot.
- Early Intervention in Airway Bleeding: In cases of hemoptysis, early intervention and management of the bleeding source (e.g., embolization or surgery) can prevent the need for emergency airway occlusion.

Implantable Cardioverter-Defibrillator (ICD)
An Implantable Cardioverter-Defibrillator (ICD) is a small electronic device implanted in the chest or abdomen to monitor heart rhythms and deliver electrical shocks when necessary to correct life-threatening arrhythmias (irregular heartbeats). It is primarily used in patients who are at high risk of sudden cardiac arrest due to ventricular tachycardia (VT) or ventricular fibrillation (VF), which are abnormal heart rhythms and this can cause the heart to stop pumping blood effectively. The ICD continuously monitors the heart’s rhythm. If it detects a rapid, abnormal heart rate, it delivers a controlled shock (defibrillation or cardioversion) to restore the heart to a normal rhythm. ICDs can also act as pacemakers, providing electrical impulses to prevent the heart from beating too slowly (bradycardia).
- Ventricular Tachycardia: This is a fast but regular heart rhythm originating from the lower chambers of the heart (ventricles). If left untreated, it can deteriorate into ventricular fibrillation (VF).
- Ventricular Fibrillation: This is a fast and chaotic heart rhythm that results in the ventricles quivering rather than contracting properly. This causes an immediate cessation of blood flow and can lead to sudden cardiac death if not treated within minutes.
- Bradycardia: In addition to its defibrillating capabilities, an ICD can also function as a pacemaker, correcting abnormally slow heart rates that can lead to fatigue, dizziness, or fainting.
The decision to implant an ICD is based on a combination of clinical evaluation, diagnostic tests, and risk factors for sudden cardiac arrest.
- ECG: An ECG records the electrical activity of the heart and helps detect arrhythmias or evidence of prior heart damage, such as from a heart attack.
- Echocardiogram: This test helps to evaluate the function of heart and its structure. A reduced ejection fraction (the amount of blood pumped out of the heart with each beat) is a key indicator for ICD implantation, especially in patients with heart failure.
- Holter Monitoring: This is a continuous ECG recording over 24 to 48 hours that can capture abnormal heart rhythms that may not be detected during a brief ECG.
- Electrophysiology (EP) Study: This specialized test maps the electrical pathways in the heart and can provoke arrhythmias under controlled conditions, helping to assess the risk of sudden cardiac arrest.
- Stress Test: A stress test may be performed to evaluate how the heart responds to physical exertion and to detect any exercise-induced arrhythmias.
The treatment process involves the implantation of the ICD device, along with its ongoing monitoring and adjustment to ensure proper functioning.
- ICD Implantation: The procedure to implant an ICD is typically performed under local anesthesia with sedation. The device is placed under the skin, usually near the collarbone, and wires (leads) are threaded through a vein into the heart. The ICD is programmed to detect and treat arrhythmias by delivering an electrical shock when needed.
- Shock Delivery: When the ICD detects a life-threatening arrhythmia such as VT or VF, it delivers a shock to restore normal rhythm. This shock can be painful, but it is life-saving.
- Pacemaker Function: In addition to treating fast arrhythmias, the ICD can also provide pacing for slow heart rhythms, helping to maintain a normal heart rate in patients prone to bradycardia.
- Follow-up Care: After implantation, patients require regular follow-up visits to check the device’s battery life, adjust programming, and ensure proper functioning of the leads and the ICD’s response to arrhythmias. Remote monitoring may also be used to track the ICD’s activity.
The prognosis for patients with an ICD is generally favorable, particularly for those at high risk of sudden cardiac death. The ICD can significantly reduce the risk of fatal arrhythmias, and most patients are able to resume normal activities after recovery from the implantation procedure.
Prognosis
- Sudden Cardiac Death Prevention: ICDs have been shown to significantly reduce mortality in patients with a history of life-threatening arrhythmias or those at high risk of sudden cardiac arrest, such as individuals with heart failure or previous heart attacks.
- Heart Failure Patients: In patients with heart failure and reduced ejection fraction, ICDs improve survival rates, especially when combined with optimal medical therapy.
- Improved Quality of Life: While the ICD itself does not treat the underlying cause of arrhythmias, it provides peace of mind for patients and caregivers, knowing that life-threatening arrhythmias can be treated automatically.
Complications
As with any medical procedure, ICD implantation and long-term use can lead to complications, some of which include:
- Infection: Infection at the site of implantation is a risk, especially shortly after surgery. It may require antibiotics or, in severe cases, removal of the device.
- Lead Displacement or Malfunction: The leads (wires) that connect the ICD to the heart can become displaced or damaged, requiring re-implantation or replacement of the leads.
- Inappropriate Shocks: Occasionally, the ICD may deliver a shock when it is not needed, such as in response to a non-life-threatening arrhythmia or due to lead malfunction. This can be distressing for the patient and may require reprogramming of the device.
- Psychological Impact: Living with an ICD can cause anxiety or depression in some patients, especially those who have experienced multiple shocks. Psychological support or counseling may be beneficial in these cases.
- Battery Life: The ICD battery typically lasts between 5 to 10 years, depending on how often the device delivers shocks. Once the battery runs low, the ICD needs to be replaced in a minor surgical procedure.
While an ICD does not prevent the development of arrhythmias, it is a critical tool in preventing sudden cardiac death. However, the prevention of the underlying causes of arrhythmias can help improve overall heart health and reduce the likelihood of ICD shocks. Key preventive strategies include,
- Heart-Healthy Lifestyle: Following a heart-healthy diet, engaging in regular physical activity, avoiding smoking, and managing stress can all help reduce the risk of heart disease and arrhythmias.
- Management of Underlying Conditions: Controlling conditions such as high blood pressure, diabetes, and coronary artery disease can help prevent the progression of heart disease and arrhythmias.
- Medication Compliance: Many patients with ICDs are also prescribed medications to manage their heart condition, such as beta-blockers, ACE inhibitors, or anti-arrhythmic drugs. Taking these medications as prescribed can help reduce the frequency of arrhythmias and ICD shocks.
- Regular Follow-up: Adhering to a regular follow-up schedule with a cardiologist ensures that the ICD is functioning properly, and any issues with the device can be detected and addressed early.
- Avoiding Triggers: Certain activities or substances can trigger arrhythmias in patients with ICDs, such as excessive alcohol consumption, stimulant drugs, or exposure to strong electromagnetic fields (e.g., MRI machines). Patients should be educated on avoiding these triggers.
